
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
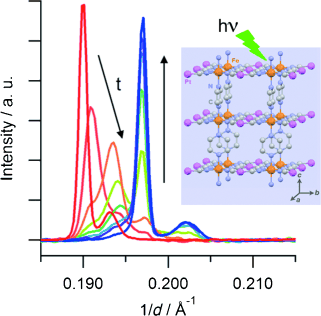
The Hoffman-type coordination compound [Fe(pz)Pt(CN)4]⋅2.6 H2O (pz=pyrazine) shows a cooperative thermal spin transition at around 270 K. Synchrotron powder X-Ray diffraction studies reveal that a quantitative photoinduced conversion from the low-spin (LS) state into the high-spin (HS) state, based on the light-induced excited spin-state trapping effect, can be achieved at 10 K in a microcrystalline powder. Time-resolved measurements evidence that the HS→LS relaxation proceeds by a two-step mechanism: a random HS→LS conversion at the beginning of the relaxation is followed by a nucleation and growth process, which proceeds until a quantitative HS→LS transformation has been reached. | ||||||||
|
||||||||
In the spin-crossover compound [Fe(6-mepy)3tren](PF6)2, (6-mepy)3tren = tris{4-[(6-methyl)-2-pyridyl]-3-aza-butenyl}amine, the high-spin state can be populated as metastable state below the thermal transition temperature via irradiation into the metal to ligand charge transfer absorption band of the low-spin species. At 10 K, the lifetime of this metastable state is only 1 s. Despite this, it is possible to determine an accurate excited state structure by following the evolution of relevant structural parameters by synchrotron X-ray diffraction under continuous irradiation with increasing intensity. The difference in metal-ligand bond length between the high-spin and the low-spin state is found to be 0.192 Ã… obtained from an analysis of the experimental data using the mean-field approximation to model cooperative effects. | ||||||||
 |
|
|||||||
In the covalently linked 2D coordination network {[Fe(bbtr)3](BF4)2}∞, bbtr = 1,4-di(1,2,3-triazol-1-yl)butane, the iron(II) centers stay in the high-spin (HS) state down to 10 K. They can, however, be quantitatively converted to the low-spin (LS) state by irradiating into the near-IR spin allowed 5dd band and back again by irradiating into the visible 1dd band. The compound shows true light-induced bistability below 100 K, thus, having the potential for persistent bidirectional optical switching at elevated temperatures. | ||||||||
|
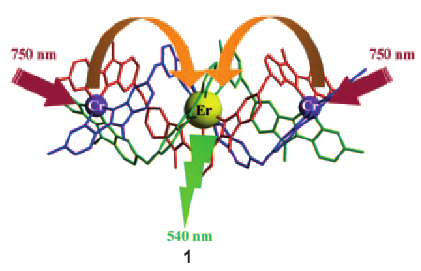 |
|||||||
The connection of two CrIII sensitizers around a central ErIII acceptor in a self-assembled cation provides high local metal concentrations that favor efficient nonlinear energy transfer upconversion luminescence (see picture). Upon selective low-energy near-infrared irradiation of CrIII-centered transitions, 1 displays an unprecedented molecular two-photon upconverted green ErIII-centered emission. | ||||||||
 |
|
|||||||
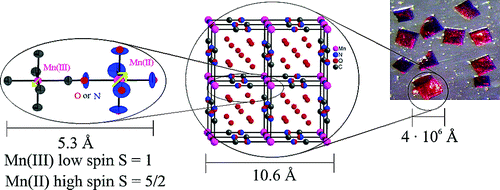
The compound of stoichiometry Mn(II)3[Mn(III)(CN)6]2·zH2O (z = 12−16) (1) forms air-stable, transparent red crystals. Low-temperature single crystal optical spectroscopy and single crystal X-ray diffraction provide compelling evidence for N-bonded high-spin manganese(II), and C-bonded low-spin manganese(III) ions arranged in a disordered, face-centered cubic lattice analogous to that of Prussian Blue. X-ray and neutron diffraction show structured diffuse scattering indicative of partially correlated (rather than random) substitutions of [Mn(III)(CN)6] ions by (H2O)6 clusters. Magnetic susceptibility measurements and elastic neutron scattering experiments indicate a ferrimagnetic structure below the critical temperature Tc = 35.5 K. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017

